The application of polyimide (PI) in the lithium battery field and the market competition landscape
Release time:
Dec 12,2024


Polyimide (PI) contributes to the realization of safe, high-performance, and long-life lithium-ion batteries as coatings, membranes, adhesives, solid electrolytes, and active storage materials.
1. Introduction to Polyimide
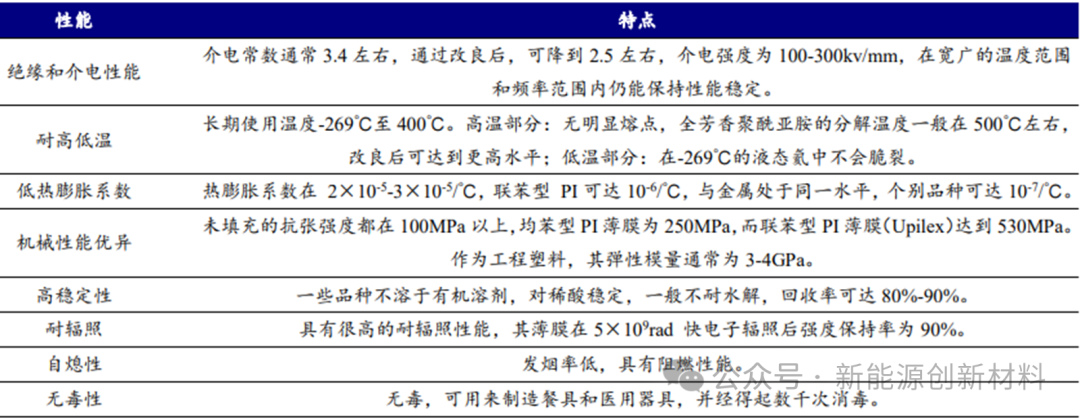
Polyimide (PI) is an aromatic heterocyclic polymer compound with a molecular structure containing imide linkages (─C(O)─N─C(O)─). It is one of the best heat-resistant varieties among engineering plastics, with a high-temperature resistance of over 400°C and a long-term use temperature range of -200 to 300°C. It is known as the top material in the polymer materials pyramid due to its outstanding characteristics in performance and synthesis. Whether as a structural material or a functional material, it has enormous application prospects and is referred to as the 'problem solver'.
2. Preparation Methods of Polyimide
The synthesis methods of polyimide mainly include hydrothermal method (one-step method) and thermal imidization or chemical imidization method (two-step method):
(1) The one-step method for synthesizing polyimide refers to uniformly mixing diamine and dianhydride monomers in a high-boiling solvent (such as m-cresol, N-methylpyrrolidone, etc.), and then directly dehydrating and polycondensing at a higher temperature (180-200°C) to synthesize polyimide, which means directly synthesizing high molecular weight polyimide without generating polyamide acid intermediates. The advantage lies in the variety of product forms, which can be made into PI films, PI powders, etc.
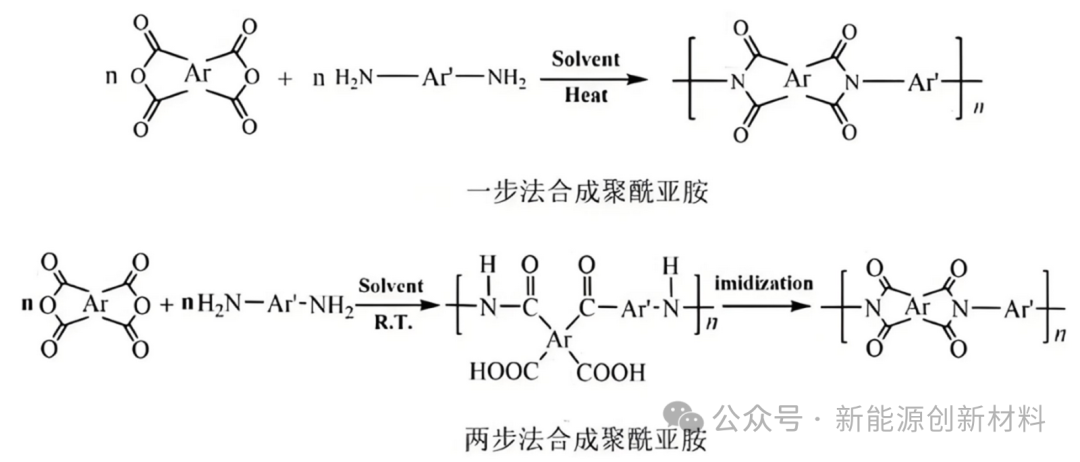
(2) The two-step method involves first synthesizing the intermediate polyamide acid (PAA) from diamine and dianhydride, and then obtaining polyimide through chemical imidization or thermal imidization. The conditions for chemical imidization are relatively mild, but the process is more complex, requiring the addition of catalysts (such as pyridine, triethylamine, etc.) and dehydrating agents (such as acetic anhydride) to complete the imidization reaction. The two-step method is suitable for large-scale preparation of PI films and is applicable for the preparation of thermoplastic PI (TPI) and thermosetting PI, allowing for the production of high-purity PI.
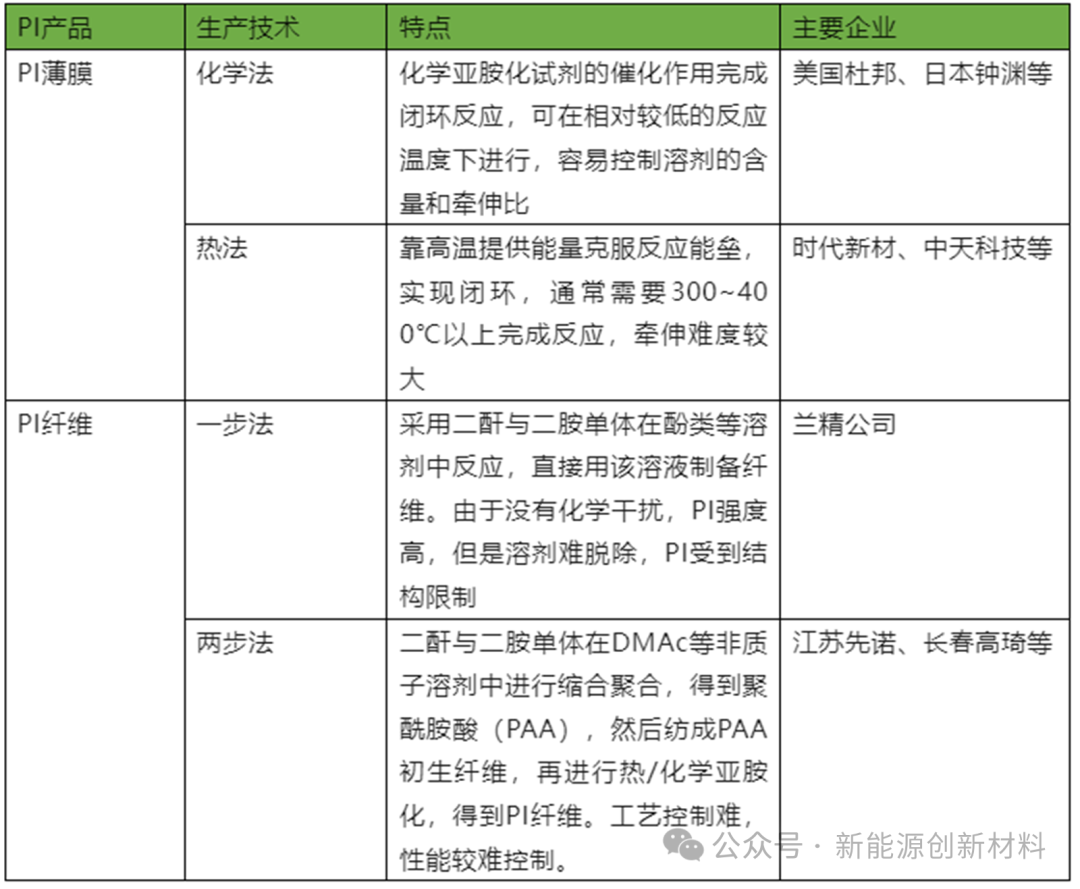
Source: 'China Chemical New Materials Industry Development Report'
3. Applications of Polyimide in Lithium Batteries
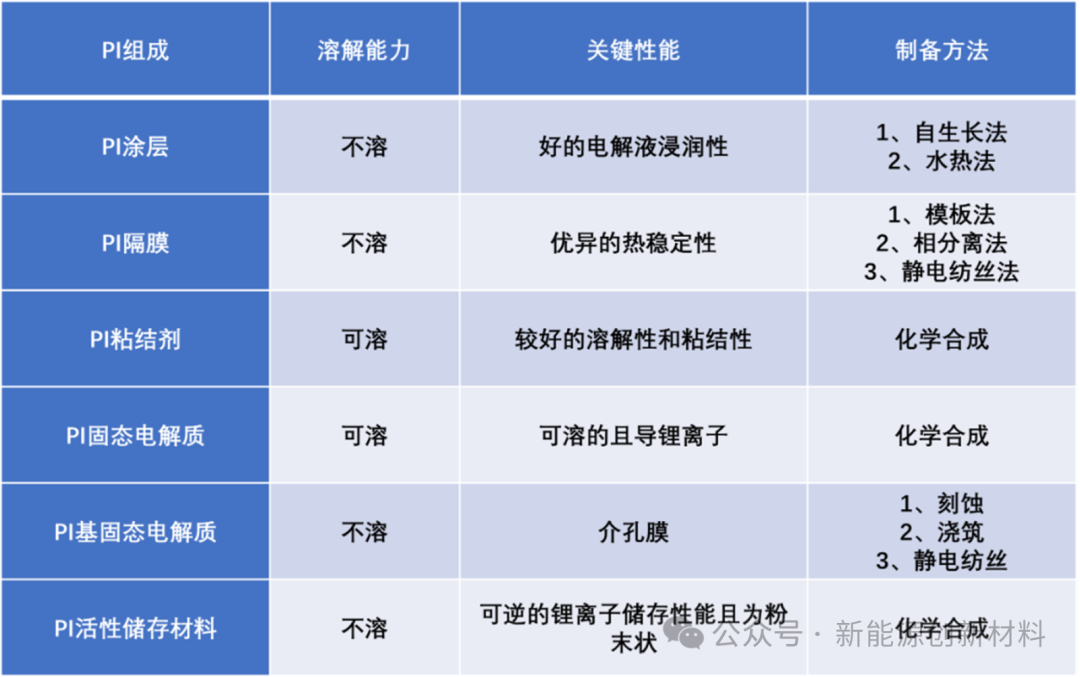
Polyimides (PIs) can be coated on the surface of active storage materials to maintain good stability of the electrode/electrolyte interface under high voltage conditions, enhancing cycling performance. PIs can be used as membranes to improve the safety of LIBs at high temperatures. To extend the cycling life of LIBs, PI binders can be used to maintain the integrity of the electrode structure. In addition, PIs can also be used as solid electrolytes or active storage materials.
3.1. As Cathode Coating Material: Enhancing High Voltage Performance
Using highly elastic, thermally stable, and well-wetted polyimide as a coating material, specific preparation methods are employed to surface-modify the cathode active material. This coating layer can suppress the volume expansion effect of the cathode during the charge and discharge process of lithium-ion batteries, while also inhibiting side reactions at the electrolyte and cathode active material interface, maintaining the stability of the crystal structure of the cathode active material. Such coating modification effectively promotes the capacity of the cathode and improves the long cycling life of the battery.
There are three application directions for PI as a coating material:
- 1) Using different monomers to synthesize PI coatings with specific properties;
- 2) Mixing inorganic materials (such as Al₂O₃, ZrO₂, TiO₂, etc.), conductive materials (such as graphene, carbon nanotubes, carbon black, etc.), and their mixtures into traditional PI coatings;
- 3) Introducing functions such as self-repair, thermal conductivity, self-extinguishing properties, and low gas emission into PIs to realize the functionalization of cathode materials.
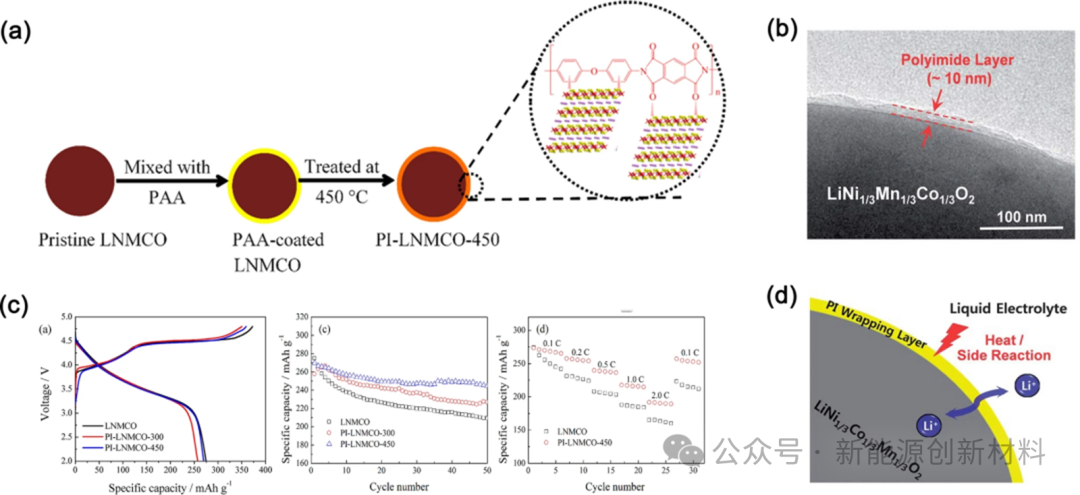
Figure: (a) Preparation method of PI-coated cathode materials; (b) TEM image of PI-coated cathode materials; (c) Initial efficiency, cycling, and rate performance of PI-coated cathode materials; (d) Coating mechanism
The PI coating layer effectively weakens the side reactions at the electrolyte and electrode surface, promotes lithium ion conduction, resulting in PI-coated cathode materials exhibiting lower polarization, reduced lithium loss, and better cycling life.
3.2. PI Membrane: Enhancing Battery Safety Performance
PI materials, with their outstanding heat resistance and good electrolyte wettability, have a significant competitive advantage in ensuring the safety of battery use at high temperatures, making them one of the most researched membrane materials. Compared to polyolefin membranes, PI membranes exhibit greater mechanical strength (greater than 10 MPa), higher thermal stability (above 500°C), lower shrinkage, and electrolyte wettability (greater than 100%).
However, the 'difficult to dissolve and melt' characteristics of PI materials limit their film-forming process, and the preparation of PI membranes is still confined to laboratories, failing to achieve mass commercialization. According to literature, the current preparation methods for PI membranes mainly include template method, phase separation method, and electrospinning method.
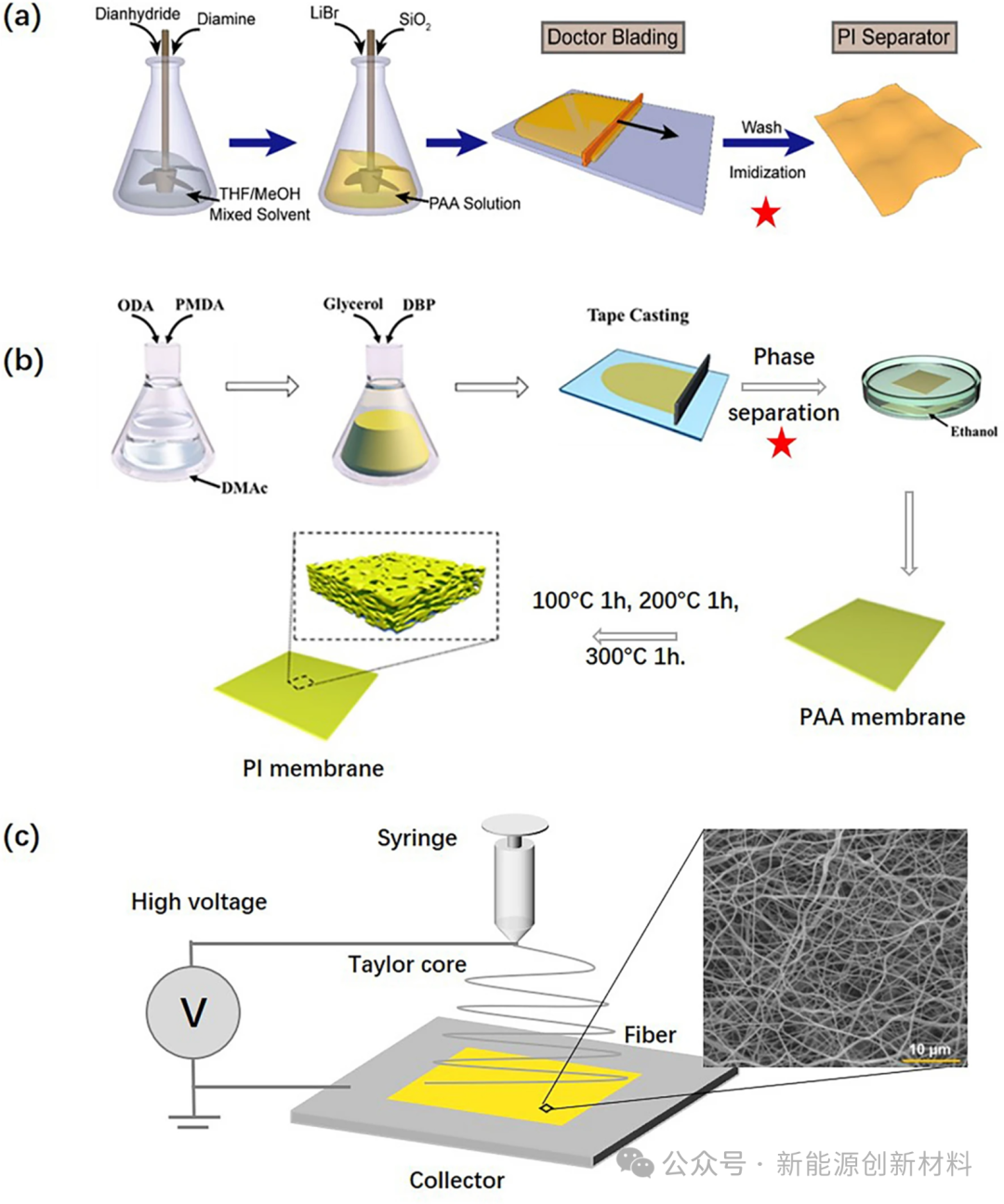
Figure: Schematic of PI membrane preparation methods: (a) Template method; (b) Phase separation method; (c) Electrospinning method.
(a) The template method requires first preparing a PI composite film containing a porogen, and then using methods such as chemical etching, solvent dissolution, or calcination to remove the porogen, resulting in a PI porous membrane.
(b) The phase separation method involves coating a polyamide acid (PAA) precursor solution or soluble PI solution onto a substrate (such as glass), immersing it in a non-solvent, and utilizing the phase separation of the polymer in its solvent/non-solvent mixed solution. After removing the solvent, the space occupied by the non-solvent forms pores.
(c) The electrospinning method applies high-voltage electrostatic force to the polymer solution. When the electrostatic repulsion on the liquid surface exceeds its surface tension, a Taylor cone forms at the nozzle. The high-speed ejected polymer solution undergoes stretching, deformation, and splitting, and as the solution evaporates, the polymer solution jet solidifies, ultimately depositing on the collector to form a nanofiber membrane.
The most critical technology in the processing is to achieve a PI membrane with uniform pore distribution. There are two strategies to solve the pore formation problem:
1) Blend PI materials with polyolefin materials to prepare composite membranes using specific processing equipment. This method utilizes the good processability of polyolefin materials to obtain PI-PE two-phase composite membranes with uniform pore distribution.
2) Use pure semi-crystalline PI materials to stretch and orient below the melting point to obtain PI membranes similar to those made by dry methods.
3.3, PI used as a binder: improving the integrity of electrode structures, new binders for silicon-based anodes.
As a binder, PI has high adhesion, good mechanical properties, excellent thermal stability, and outstanding electrolyte wettability, which can maintain the structural integrity of the electrode, especially in materials like silicon-based anodes that experience significant volume expansion and contraction. The 3D cross-linked network structure of the PI binder can effectively constrain active particles, preventing silicon particle pulverization and loss of electrical contact, greatly maintaining the integrity and stability of the silicon anode structure, thereby improving the cycling stability of the battery.
Currently, many research works have reported PI binders from the perspective of molecular structure design, aiming to improve their specific properties. Introducing ether oxygen functional groups can enhance the lithium ion conductivity of PI binders; synthesizing polymers with soft and hard segments can endow PI binders with better elasticity; strong adhesive functional groups can be introduced into the molecular structure of PI to enhance the adhesion performance of PI binders.
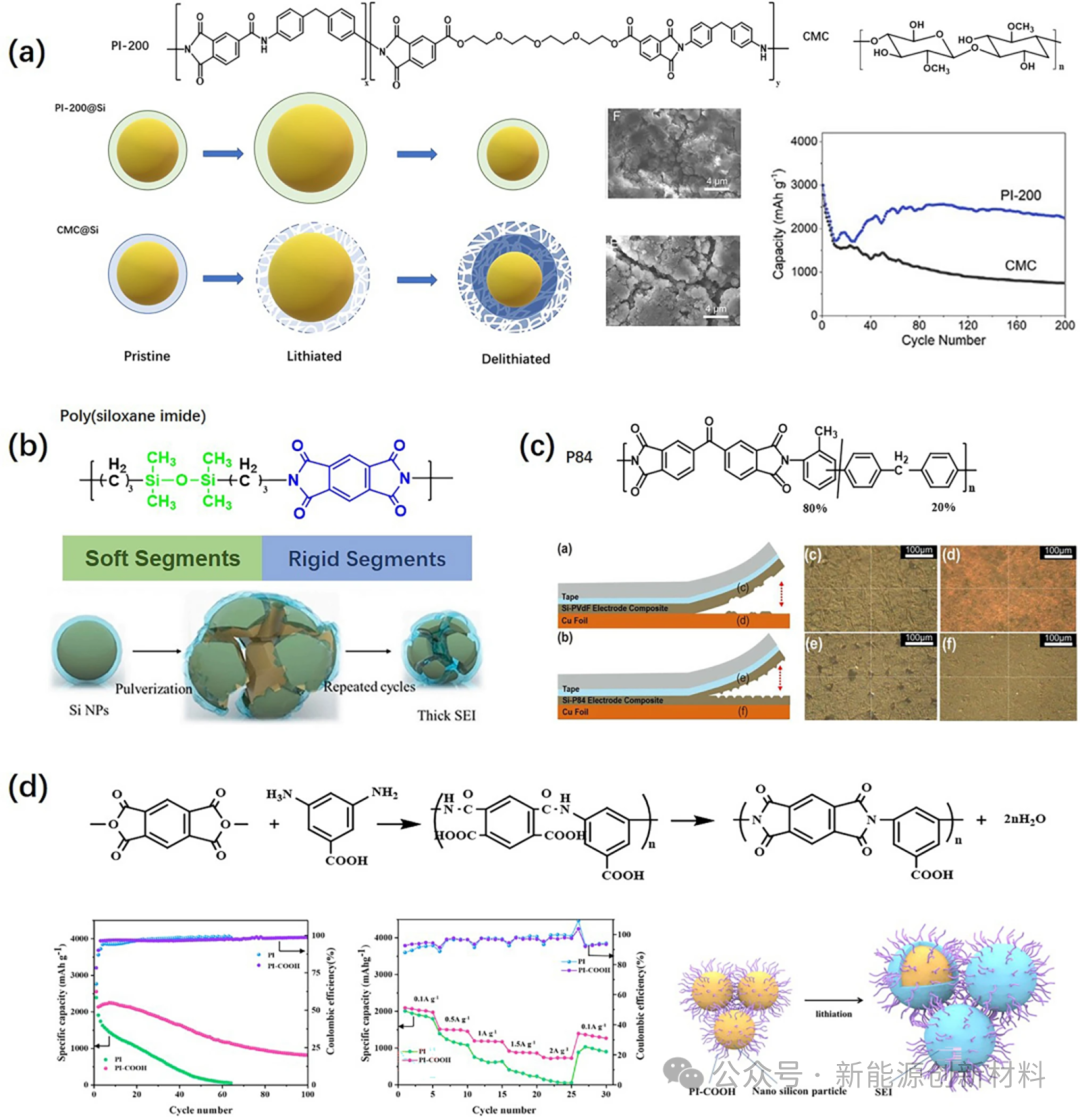
Figure: Application of PI modified binders on silicon-based anodes: (a) PI modified binders obtained from the polymerization of PEG-200, trimellitic anhydride chloride (TMAC), and 4,4'-diaminodiphenyl ether (MDA) during the cycling process, showing volume expansion, cycling performance curves, and SEM images of the electrode after cycling; (b) PI binders with soft and hard segments; (c) Schematic diagram of the peeling effect of P84 vs PVDF binders; (d) Structure, electrochemical performance, and SEI formation mechanism of PI-COOH binders.
3.4, PI-based solid-state electrolytes: all-solid-state lithium-ion batteries.
Polyimide (PI), benefiting from its excellent mechanical strength, good electrolyte wettability, and outstanding thermal stability, makes PI-based solid-state electrolytes an ideal choice for all-solid-state lithium-ion batteries.
(1) The high mechanical strength of PI helps improve the overall stability and durability of the electrolyte, which is crucial for enhancing the safety and lifespan of the battery.
(2) The electrolyte wettability of PI helps ensure good contact between the electrolyte and the electrode, thereby improving ion transport efficiency.
(3) The excellent thermal stability of PI means that the electrolyte can maintain stable performance in high-temperature environments, which is significant for enhancing the safety and reliability of the battery under extreme conditions.
In terms of application, the use of PI-based solid-state electrolytes in all-solid-state lithium-ion batteries is mainly reflected in the following aspects:
(1) As mechanical support:By using ultra-thin polyimide porous membranes as mechanical support, three-dimensional continuous lithium conduction channels can be formed, thereby improving the mechanical properties and electrochemical stability of the solid electrolyte.
(2) Enhancing battery safety:By designing and utilizing strategies, PI-based solid-state electrolytes help achieve safe, high-performance, and long-lifespan lithium-ion batteries.
(3) As solid-state electrolytes:In all-solid-state lithium-ion batteries, PI-based solid-state electrolytes exhibit good cycling stability and high safety performance.
(4) Enhancing battery performance:By adding flame-retardant particles and optimizing the electrolyte structure, PI-based solid-state electrolytes can significantly enhance the safety and performance of the battery.
Generally, PI solid-state electrolytes can be divided into solid-state PI electrolytes and solid-state PI-based electrolytes. Solid-state PI electrolytes are formed by introducing PEO molecular fragments into the PI main chain, while solid-state PI-based electrolytes involve introducing PEO into mesoporous PI membranes. Currently, only a few studies directly use solid-state PI electrolytes. Most research works use solid-state PI-based electrolytes. Additionally, solid-state PI-based electrolytes can be further divided into solid-state PI-based solid polymer electrolytes and solid-state PI-based composite solid electrolytes.
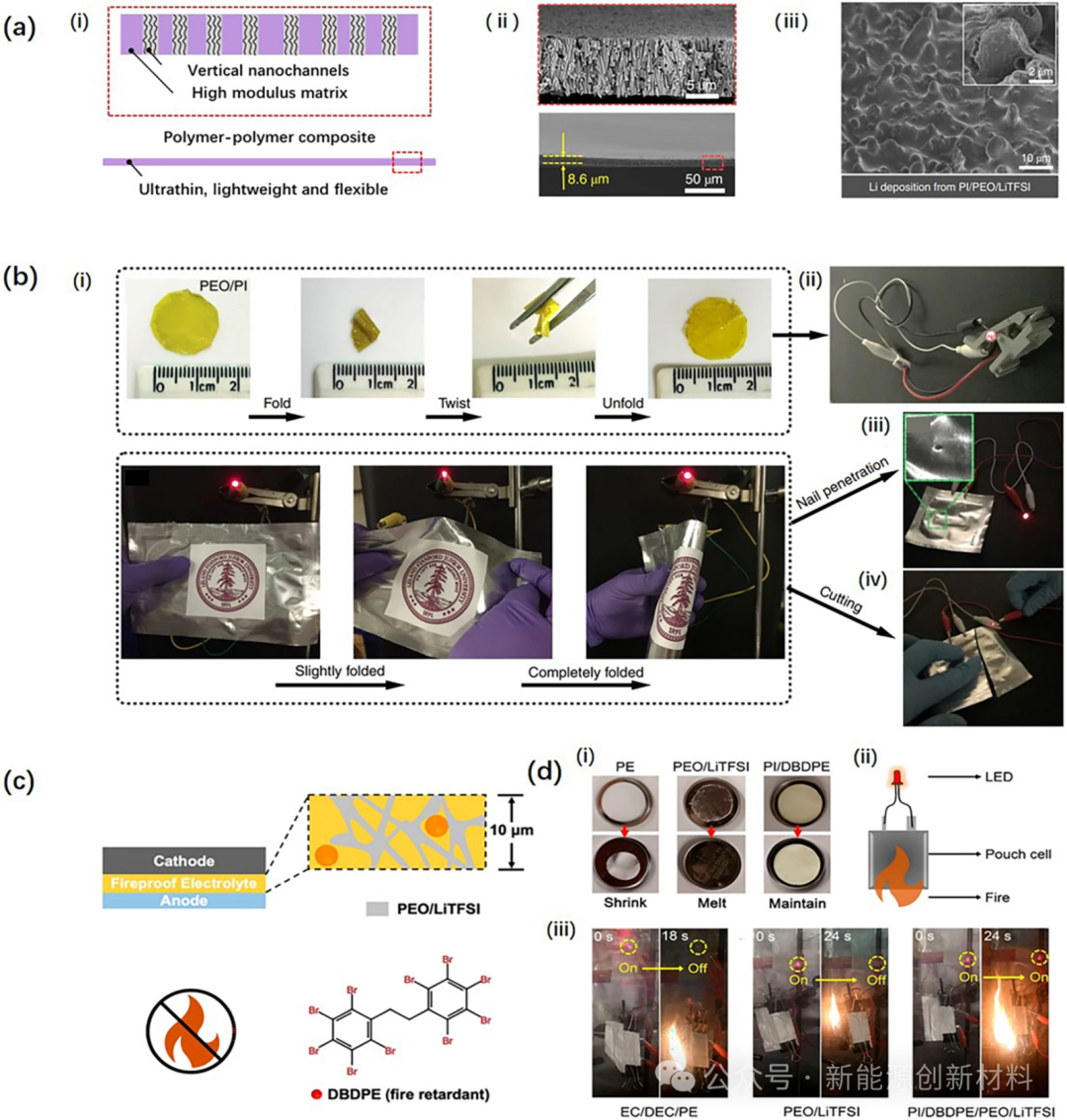
Figure: Application of PI-based solid-state electrolytes in all-solid-state lithium-ion batteries: (a): (i) Vertically etched nanopores on PI membranes, (ii) the resulting ultra-thin, lightweight, and flexible PI solid polymer electrolyte, (iii) uniform lithium deposition layers observable by SEM after cycling. (b) Abuse testing of PI/PEO/LiTFSI-based LIBs: (i) Folding performance; (ii) button cell lighting an LED bulb; (iii) flexible Li/PEO/LiTFSI/LFP pouch cell lighting an LED bulb; (iv, v) puncture and cutting tests. (c) Flame-retardant PI SPE with added flame retardant DBDPE; (d) Thermal abuse testing: (i) PE separator, PEO/LiTFSI, and PI/DBDPE membranes placed at high temperatures (150°C for 0.5 h); (ii) Schematic diagram of pouch cell thermal abuse; (iii) Abuse testing of batteries with electrolytes EC/DEC/PE, PEO/LiTFSI, PI/DBDPE/PEO/LiTFSI.
3.5, PI active storage materials: next-generation organic electrodes.
Polyimide PI belongs to carbonyl polymers and is an organic electrode material with high capacity, good cycling and rate performance, as well as adjustable structure, safety, and environmental friendliness.
(1) As positive electrode active storage materials.The lithium-ion storage mechanism is based on the reversible redox reaction of carbonyl groups on the repeating units of PI molecules, primarily occurring at 1.5~3.5V. Currently, conjugated structures, reducible carbonyls, and ether oxygen fragments are the main structural units for regulating electrochemical performance. Additionally, designing suitable stacking structures can also achieve rapid storage and transport of lithium ions.
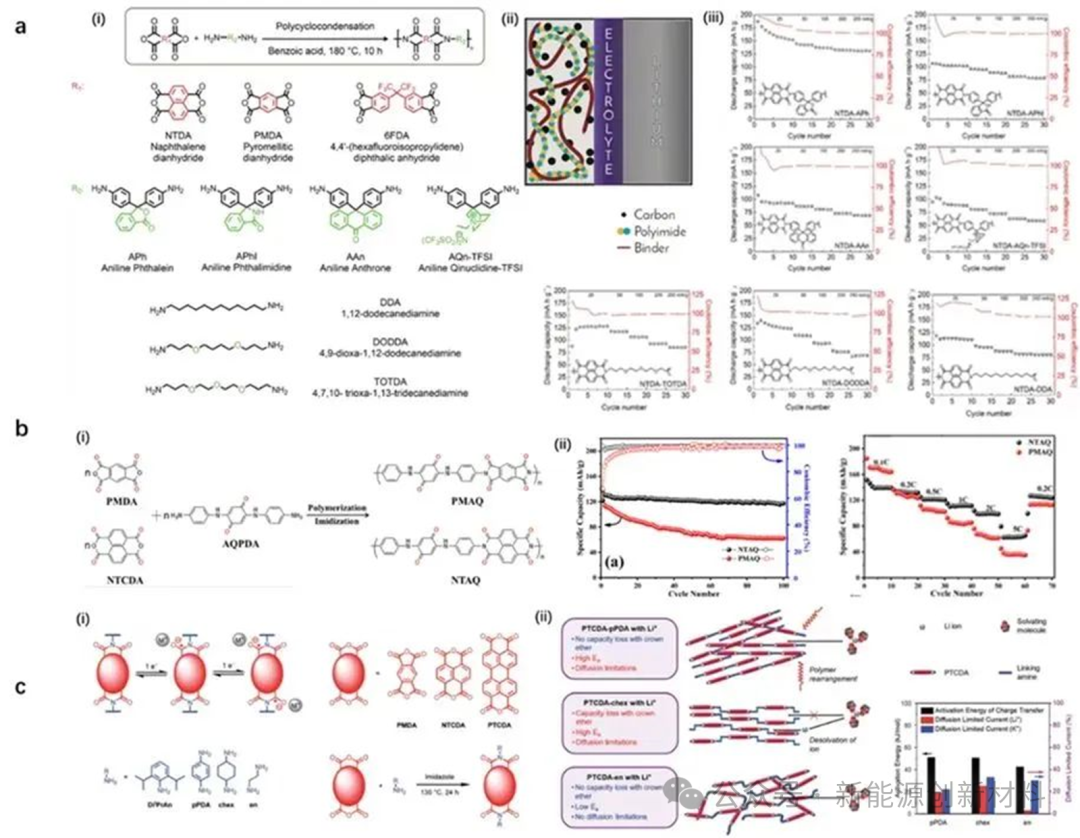
Figure: (a, b) Introduction of conjugated carbonyl and ether oxygen bonds in PI cathode active materials; (c) Stacking structure design enhances lithium ion conductivity.
(2) When PI active storage materials are used as anode, the lithium storage mechanism can be described as 'super lithiumization'.The operating voltage range is 0~1V. Introducing more benzene rings into the PI molecular structure is beneficial for lithium ion storage. To further enhance cycling and rate performance, different conductive materials such as graphene, carbon nanotubes, and carbon black are compounded with PI active storage materials to prepare electrodes.
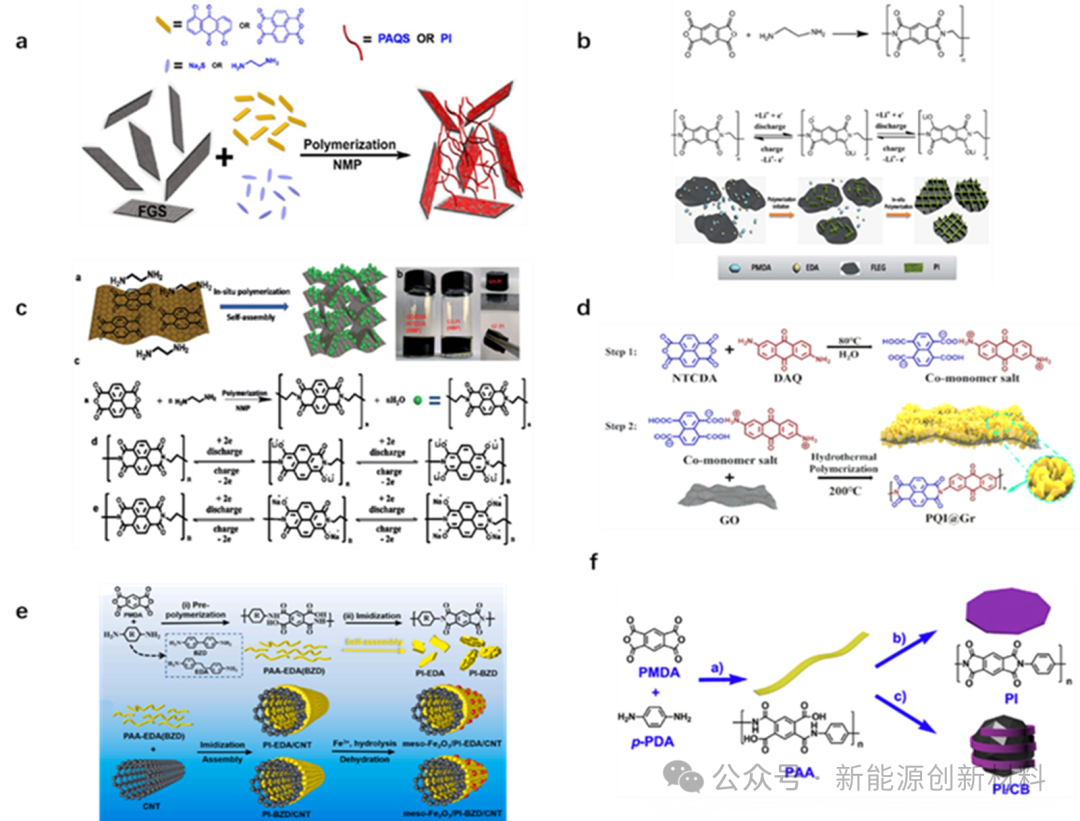
Figure: Anode materials prepared by compounding graphene, carbon nanotubes, carbon black, etc. with PI active storage materials.
IV. Polyimide Market and Competitive Landscape
4.1. Market Size
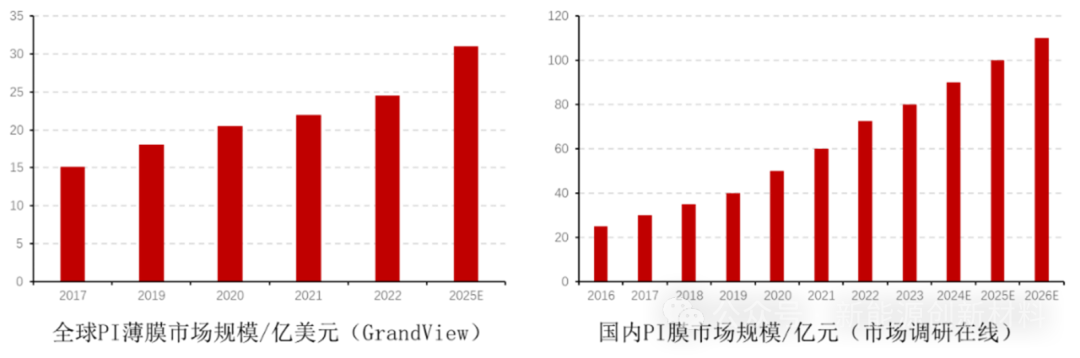
The global polyimide film market is continuously expanding. With the upgrade of consumer electronic products, the requirements for material heat dissipation performance, dielectric performance, etc. are increasing. The rapid development of flexible display technology accelerates the development of the PI market. In 2022, the total global PI film market size was $2.45 billion, and GrandView expects the global PI film market to grow to $3.1 billion by 2025. According to data from Market Research Online, the total domestic PI film market size reached 7.24 billion yuan in 2022, including 2.63 billion yuan for electronic-grade PI films, 2.69 billion yuan for special-grade PI films, 950 million yuan for thermally conductive PI films, and 970 million yuan for electrical-grade PI films; it is expected to reach 11 billion yuan by 2026. The growth rate of China's PI industry is far higher than the global level, with the overall market size of the PI industry expected to exceed 20 billion yuan by 2024, and the CAGR from 2019 to 2024 is expected to reach 8.5%.
4.2. Competitive Landscape
Currently, the global PI market is still dominated by the United States and Japan, accounting for about 60% of the global market share. Major companies such as DuPont in the United States, Ube Industries, Zhongyuan Chemical, Toray Group, Mitsui Chemicals, and Mitsubishi Gas Chemical in Japan hold core technologies for PI films, resins, slurries, etc. Europe and South Korea are in the second tier, accounting for about 20% of the global market share, with major companies including Evonik in Germany, Solvay in Belgium, and SKC in South Korea.
Polyimide material products can be divided into various forms such as films, fibers, and foams; films are the largest application market for polyimide, which can be divided into electrical grade, electronic grade, thermal control grade, etc.; domestic large-scale production of electrical grade films has been achieved, but electronic grade films still rely on imports, with an import dependency of 80%. The main reason is that core technologies such as slurry synthesis and formulation processes are controlled by foreign companies, resulting in a very low domestic production rate of slurries.
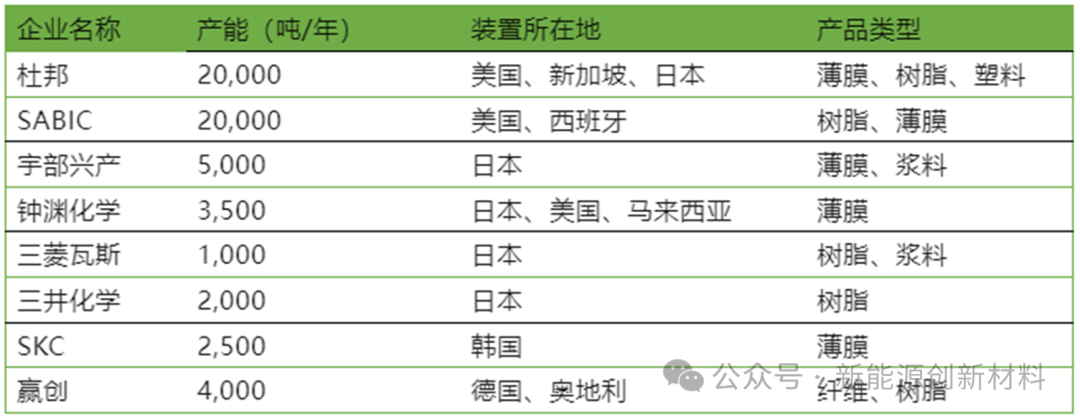
(1) International PI Enterprises' Production Capacity
Currently, the global PI market is still dominated by the United States and Japan, accounting for about 60% of the global market share. Major companies such as DuPont in the United States, Ube Industries, Zhongyuan Chemical, Toray Group, Mitsui Chemicals, and Mitsubishi Gas Chemical in Japan hold core technologies for PI films, resins, slurries, etc. Europe and South Korea are in the second tier, accounting for about 20% of the global market share, with major companies including Evonik in Germany, Solvay in Belgium, and SKC in South Korea.
(2) Domestic PI Enterprises' Production Capacity
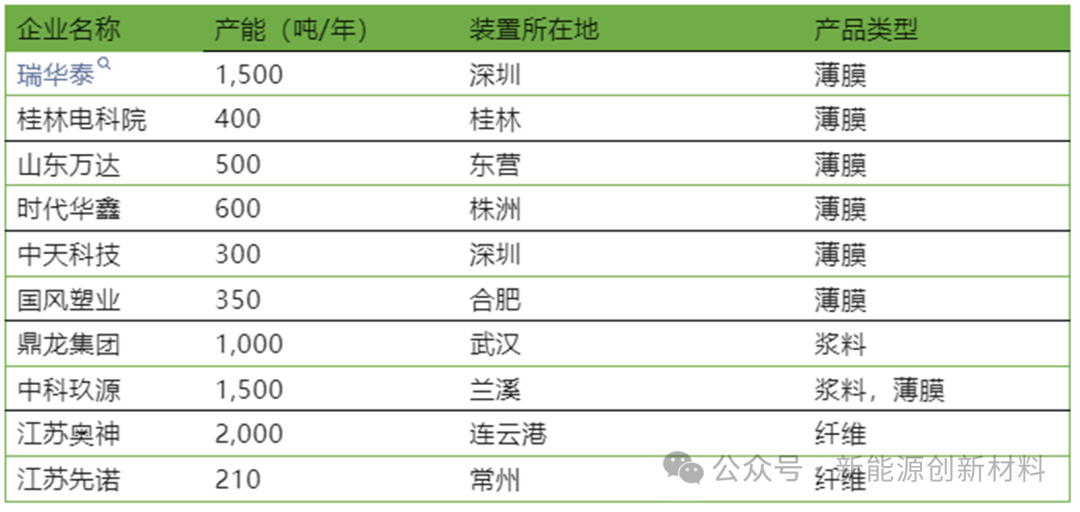
The domestic polyimide industry started relatively late but has developed rapidly in recent years. Domestic companies such as Changchun Gaoki, Shenzhen Ruihuatai, Guilin Electric Institute, and Jiangsu Aosheng have made significant progress in the fields of PI films and fibers.
Currently, there are dozens of domestic companies such as Guilin Electric, Shandong Wanda Microelectronics, Zhuzhou Times, and Shenzhen Ruihuatai that have the production capacity or plans to produce PI films. However, due to the limited development level of raw materials, equipment, and other links in China, the manufacturing level of high-end PI films is still in the early stages of development.
More information
The list of the top 100 counties for 2024 has been released! Rudong is ranked this time...
On December 6, the National County Economic Research Institution - Zhongjun Research Institute completed and released the "24th County Economic and County Development Monitoring and Evaluation Report."
Dec 12,2024
The application of polyimide (PI) in the lithium battery field and the market competition landscape
Polyimide (PI) serves as a coating, membrane, adhesive, solid electrolyte, and active storage material, contributing to the realization of safe, high-performance, and long-lasting lithium-ion batteries.
Dec 12,2024
Advantages of the Yangkou Chemical Industrial Park
Yangkou Chemical Industrial Park is located in Rudong County, Jiangsu Province, built on the vast reclaimed tidal flats of Rudong County. It is 35 km from the county seat, 60 km from Nantong City, and 130 km from Shanghai, with a distance of less than 5 kilometers to nearby rural residential areas. It is situated in the most economically active Yangtze River Delta region of China, at a suitable distance from the city.
Dec 12,2024
Public Announcement of Clean Production Audit
In order to improve resource utilization efficiency, reduce and avoid pollutant generation, and promote enterprises' synergistic effects of pollution reduction and carbon reduction, the government has issued relevant notices requiring enterprises to carry out clean production audits.
Apr 11,2022

 中文
中文
